In 2002, Iranian supreme leader Ayatollah Ali Khamenei issued a religious ruling, a fatwa, declaring embryonic stem cell research acceptable under Islamic law. American scientists at that time were stuck in an epic political debate over the cells’ use, but Iranian researchers had a green light to launch various experiments, develop cell lines, and invent novel therapies.
In the 14 years since, they’ve made great strides in stem cell research. And now that Iran is losing its pariah-state status after sanctions were lifted in mid-January, there are opportunities for collaborations with non-Iranian scientists—which has Ali Brivanlou, who leads the Stem Cell Biology and Molecular Embryology lab at The Rockefeller University, intrigued about the possibilities.
Brivanlou discussed the state of stem cell research in Iran—and what other scientists might learn from that research—during a recent presentation at the American Association for the Advancement of Science's annual meeting in Washington, D.C. Born in Tehran, Brivanlou did his post-doctoral research at the University of California, Berkeley, and continued his career in the United States. When sanctions were lifted, he visited Tehran again. “After 36 years, I was quite impressed with what I saw,” he said during his talk. “Iran is certainly at the cutting edge of stem cell research, in terms of basic knowledge and in terms of application platforms.”
Brivanlou later spoke to mental_floss about the potential benefits of collaborating with Iranian scientists. So too did panel organizer Richard Stone, who oversees international coverage at the journal Science. Stone noted to mental_floss that before the sanctions were lifted, there were too many constraints for any American-Iranian scientific collaborations to really work. “That turned scientists off,” Stone said. Now new possibilities are opening up.
THE SCIENCE—AND POLITICS—OF STEM CELL RESEARCH IN THE U.S.
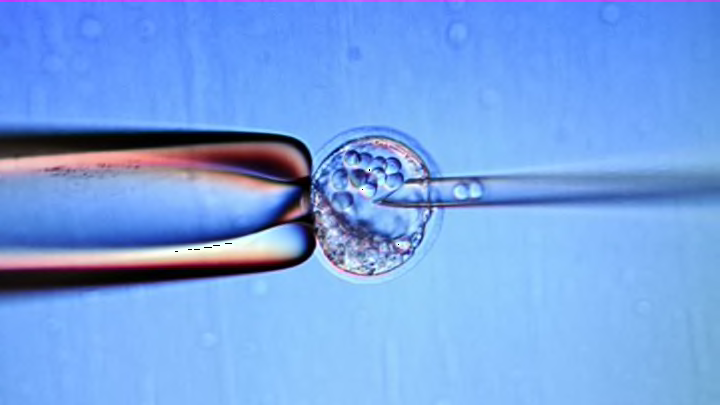
Derived from a fertilized human egg, embryonic stem cells are pluripotent—under the right conditions, they can develop into any of the 200 cell types present in the body of an adult. Under certain other conditions, they can keep replicating themselves forever. All these unique qualities make embryonic stem cells extremely valuable not only for basic research, but also for a gamut of medical cures—from regenerative medicine to tissue replacement therapies to treating genetic diseases.
But because embryonic stem cell usage implies that, at some point, some embryo had been destroyed to harvest its cells, this research spawned a great deal of controversy in the Western world.
When the Bush administration placed various restrictions on the cells’ usage and funding in 2001, American embryonic stem cell researchers found themselves in the crosshairs of ethical, religious, and funding wars. For the next several years, politicians, lawyers, and advocacy groups wrote letters, signed petitions, and composed bills—some in favor of the practice, others against it. Bills were passed by Congress and vetoed by President George W. Bush, until in 2009 President Obama lifted the restrictions, expanding the number of stem cell lines that qualified for federally funded research.
THE SCIENCE—AND POLITICS—OF STEM CELL RESEARCH IN IRAN
Meanwhile, the Royan Institute in Tehran, a city of nearly 9 million people on the slopes of the Shemiran Mountains, was an embryonic research safe haven. (Royan means "embryo" in Farsi.) Iran didn’t view stem cell research as problematic because under Islamic law life is defined not at conception, but when one can distinguish a heartbeat, Brivanlou explained in his talk.
Royan scientists began operating embryonic cell lines in 2003, and now have over 40 different lines in clinical trials, Brivanlou told mental_floss. In 2006, they successfully cloned a sheep, naming it Royana, and last year they cloned an endangered animal—an Isfahan mouflon (a wild sheep). “This was their tour de force,” he said at the conference. “It was a nucleus of a mouflon grown inside a sheep.”
While the world scrutinized Iranian nuclear advances, the country’s stem cell embryonic research had risen to the scientific forefront.
FROM 12 TO 362 STEM CELL LINES SINCE 2004
For the past few years, stem cell research in the U.S. has made a lot of progress, David Schaffer, director of the Berkeley Stem Cell Center, told mental_floss. Schaffer studies stem cell bioengineering and its applications in regenerative medicine. “We now have 362 lines on the federal registry compared to something like a dozen in 2004,” he said.
Scientists in the U.S., often in collaboration with researchers in Europe and Japan, have managed to grow muscles, bones, kidneys, intestines, and liver and heart tissue from stem cells, aiming to treat disease or alleviate the shortage of donor organs. There are clinical trials underway to treat degenerative eye disease with retinal cells derived from stem cells. The goal of another trial is to alleviate spinal cord injuries by growing myelinated cells, which serve as neuron insulators. Schaffer’s lab is looking into the possibilities of regenerating brain cells that die off in Parkinson’s disease.
Partnering with Iranian colleagues offers many advantages, Brivanlou said. The Iranian scientists, who worked in isolation from the rest of the world, experimented in different research areas—such as cloning endangered species to prevent their extinction. (Besides the mouflon, they’re also working on potentially cloning an endangered white tiger that lives in the mountains of Iran.) They focused on finding ways to treat region-specific infectious diseases and genetic disorders caused by inbreeding. They also focused on producing antidotes to local venomous snakes such as cobras. These technologies can help countries neighboring Iran, which face similar medical and environmental challenges but aren’t as advanced.
LIMITED RESOURCES LED TO SCIENTIFIC CREATIVITY
It’s important to note, Brivanlou said, how much Iranian scientists were able to achieve with the rudimentary tools they had. He likens it to building a car without having hammers and screwdrivers at hand. Bioreactors that grow stem cells are complex pieces of equipment— computer-controlled to feed nutrients to cells, remove cellular waste, and keep cultures at precise temperature. Reagents used to grow cells are specific chemical solutions that Western labs buy from companies that make them.
Sequencing DNA, which is part of stem cell research, requires high-end robotics and various chemical solutions. Brivanlou’s lab can order a dozen reagents from around the world and they get shipped by FedEx the next day. But many Western biochemical companies couldn’t sell products to Iran, and there’s still no FedEx delivery, so Iranian scientists have had to make everything from scratch.
A bioreactor Brivanlou saw in Iran looked as if it was made in someone’s garage. “It was just a metal chamber with a couple of tubes and a burning candle underneath to keep it at the right temperature—but it worked and it grew cells,” Brivanlou recalled. “An experiment that takes me a week to make would take an Iranian scientist a year. Imagine what they could accomplish if they had the same access we do.”
Stone also said that because Iranian scientists had to play by tougher rules, they learned to think about every little detail of a study or experiment. Repeating experiments was difficult and costly, so they learned to anticipate what a paper reviewer might ask for—and plan for it. “That allowed them to be competitive in a very tough research field,” Stone said. “It made them better scientists.”
Joining forces in research would unlock the untapped potential the Iranian stem cell scientists hold, Brivanlou said. It would also allow Western and Iranian scientists to share and exchange research materials, allowing for greater genetic diversity in experiments.
Brivanlou hopes to begin collaborating soon, starting by Skype and expanding to other venues: “My dream is to have universities in the United States, such as The Rockefeller University, and institutes in Iran, such as the Royan Institute, to be engaged in a double exchange program as soon as possible,” he said at the conference.