Caught on Camera: An Amoeba Eating Human Cells Alive
Are you squeamish? Does the thought of germs and/or microscopic organisms give you the creeps? You might want to turn back now.
The Entamoeba histolytica amoeba infects 50 million people all over the world, and every year, it kills roughly 100,000 of them. Until recently, researchers weren’t sure how this microbe worked. Now, thanks to postdoctoral researcher Katherine Ralston and her team at the University of Virginia in Charlottesville, we have some unsettling insight into how the amoeba attacks its victims: It eats their cells, bit by bit, until the cells are dead.
This particular infection occurs most commonly in developing nations, after victims ingest contaminated food or water. In some parts of Bangladesh, for example, Ralston says 30 percent of children contract the amoeba at least once before their first birthday. While some victims show no symptoms at all, in others, it can cause severe dysentery and even spread to other organs—like the liver—which can be deadly. So, researchers wanted to know: What exactly happens after these microbes are ingested? How do they go on to cause disease in other parts of the body?
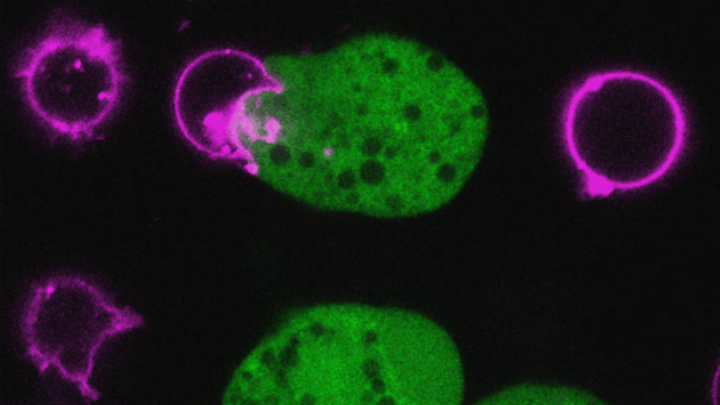
To get an up-close look at the action, researchers, led by Virginia’s William Petri, Jr., mixed the amoeba with human cells and observed its behavior under a microscope. Like a microscopic Pacman, almost immediately upon contact, the parasite started taking bites of and ingesting the human cells. Within about 10 minutes, the cells met their untimely death, and the amoeba moved on to its next victim. Researchers caught the whole thing on video.
Why is this process so shocking? Aside from the cringeworthy fact that a parasite is slowly eating human cells alive, researchers were also surprised because many other microbes take a somewhat less violent approach to eliminating cells, emitting something toxic to poison them. “It was thought they would kill cells like a lot of other microbes kill cells,” Ralston told mental_floss. “Instead you see them gnawing on the cell. This really physical cell death is not typical.”
Even more puzzling: The parasite isn’t using the human cells as a primary source of nourishment. “Once these cells had been killed, they stopped eating and moved on to a new cell,” Ralston says. “If it was about nutrition, they would ingest the rest of the cell as a readily available meal.” So why go through the trouble of ingesting the cells, if not for nourishment? Ralston and her team think this is a way for the parasite to invade the intestine, or perhaps a defense mechanism against immune cells sent to rescue the victim from the parasite.
But researchers needed to be sure that this gnawing process is what actually kills the cells. Could cells survive if they’d only been bitten once or twice? If they knew the cause of cell death, they could move forward developing treatments to prevent it. So they altered the microbe, inhibiting its ability to attach to the cells, and saw that indeed, the cells can endure the carnage, up to a point. “We actually found that with some inhibitors—even if we didn’t completely inhibit nibbling, just reduced it—that it allowed the human cells to live,” Ralston says. “It seems there’s a threshold. If we could block this process, that would be a potential new therapy.”
The findings appear in the April edition of the journal Nature and could lead to new treatments for Entamoeba histolytica, which is currently treatable with one class of drugs. “This changes the paradigm for this infection, “ Ralston says.
Live confocal microscopy time lapse demonstrating that bites of human cell material are internalized by the amoebae. Ingestion of bites occurs while human Jurkat cells are viable and ceases once they are dead. Human cells were pre-labeled with DiI (red; cell membrane), Flou4 (green; intracellular Ca2+); and SYTOX blue (blue; nucleic acid in permeable cells) was present in the media during imaging. Images were collected every 30 seconds and are played back at 1 frame per second
Live confocal microscopy time lapse demonstrating that human cell intracellular calcium elevation follows the ingestion of bites. Human Jurkat cells were pre-labeled with DiD (pink; cell membrane), and Flou4 (green; intracellular Ca2+). Images were collected every 20 seconds and are played back at 1 frame per second.